THE PROCESSES
Alcohol production through distillation in France
Alcohol production through distillation in France dates back to the late 13th century, when it was a privilege reserved for master vinegar-makers. This monopoly was abolished by Louis XIII, who officially recognized the profession of distillers and makers of spirits.
At the beginning of the 19th century, the invention of the continuous distillation apparatus by Adam revolutionized production. In 1854, Mr. Champonnois created the first beet distillery in France, marking the beginning of a rapid expansion in ethyl alcohol production.
A bit of (bio)chemistry…
Simplified chemistry of transforming glucose into alcohol from plant-based materials:

The stages of bioethanol production
Bioethanol production is broken down into several essential stages.
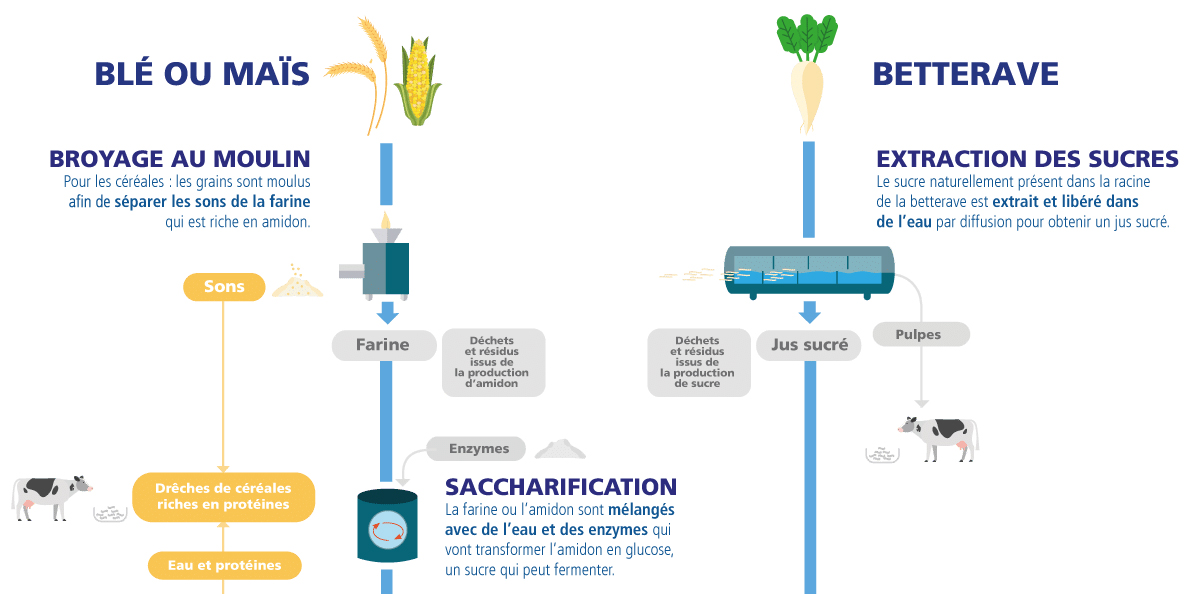
1) Extraction of fermentable sugars
Beet : The sugar naturally present in the beetroot is extracted and released into water through diffusion, along with other soluble substances, to obtain a sugary juice.
Cereals: The grains are dry-milled to separate the bran (cellulosic husks) from the flour, which is rich in starch and also contains proteins. The flour is then mixed with water and enzymes that convert the starch into glucose, a sugar that can ferment. This corresponds to the saccharification step. In the specific case of a starch plant, the grains are “wet-milled,” with water, to separate the starch from the other components.
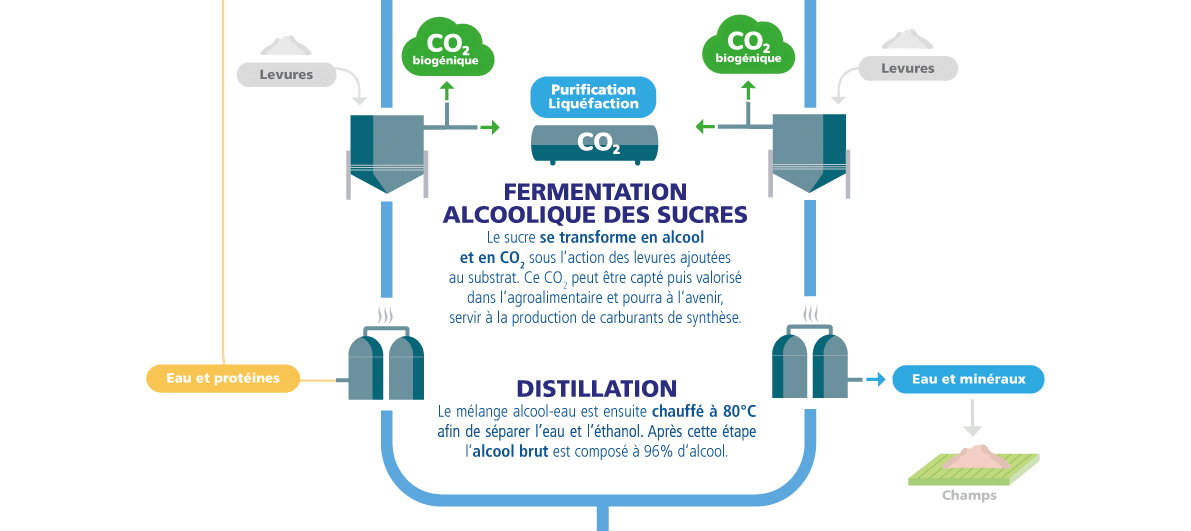
2) Fermentation and Distillation
Fermentation
The sugar is converted into alcohol and CO₂ through the action of yeast added to the substrate. This CO₂ can be captured and reused in the agri-food industry and, in the future, could be used for the production of synthetic fuels.
Distillation
The alcohol–water mixture is then heated to 80°C to separate the water from the ethanol. After this step, the crude alcohol contains 96% alcohol.
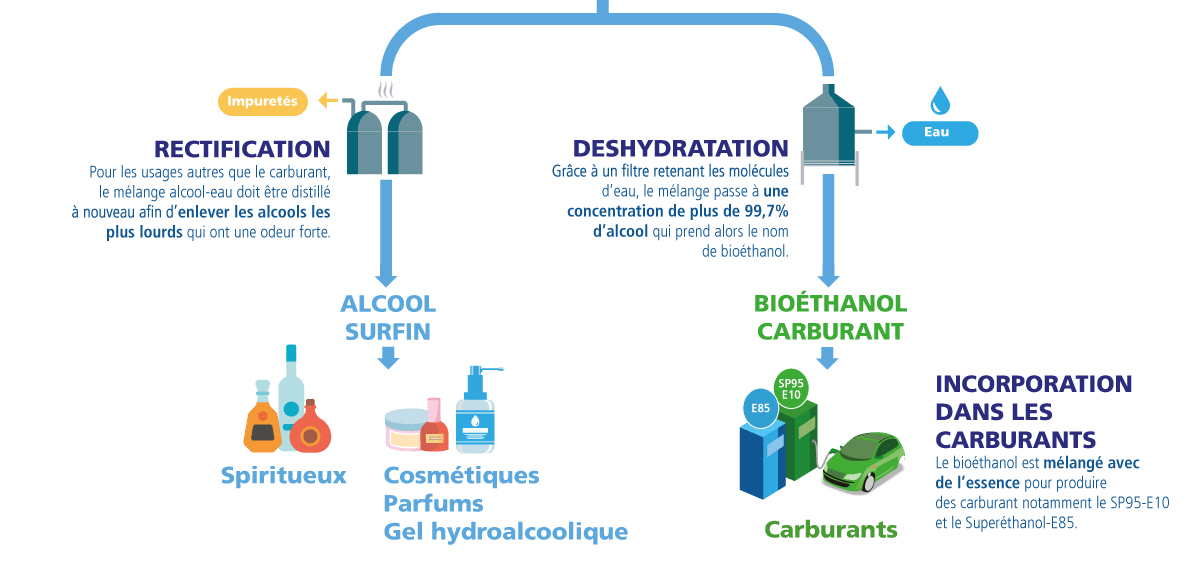
3) Rectification and Dehydration
Rectification
For uses other than fuel, the alcohol–water mixture must be distilled again (rectification) to remove the heavier alcohols that have a strong odor. Finally, “extra-fine” alcohol is obtained, which can be found in spirits as well as in cosmetics (perfumes, hydroalcoholic gel).
Dehydration
Thanks to a filter that retains water molecules, the mixture reaches a concentration of more than 99.7% alcohol, which is then called dehydrated bioethanol. This produces a bioethanol that can be blended into fuels. Bioethanol is mixed with gasoline to produce fuels, notably SP95-E10 and Superethanol-E85.